Diovan blood pressure medication recall
FDA Warns Sixth Valsartan Blood Pressure Drug Recalled for Contamination
Now, generics maker Mylan Pharmaceuticals is adding more products to the list. The recall involves lots of three medicines: The reason for the move is medication recall to that cited in prior valsartan recalls: Mylan said it had detected trace amounts diovan blood pressure blood pressure medication recall a probable cancer-causing chemical called N-nitrosodiethylamine NDEA in valsartan meds.

Food and Drug Administration is currently trying to trace the source of the contaminants, originally linked to factories in China and India that help supply valsartan to generic drug makers. Those factories were linked to contamination diovan blood pressure medication recall a diovan blood pressure medication recall type of potential diovan blood pressure medication recall, called N-nitrosodimethylamine, or NDMA.
Another Blood Pressure Medication Being Recalled — Here’s What You Need to Know
diovan blood pressure medication recall Valsartan is used to treat high blood pressure, heart failure, and to reduce the risk of death after a heart attack. Valsartan in combination with amlodipine or hydrochlorothiazide is used to treat high blood pressure.
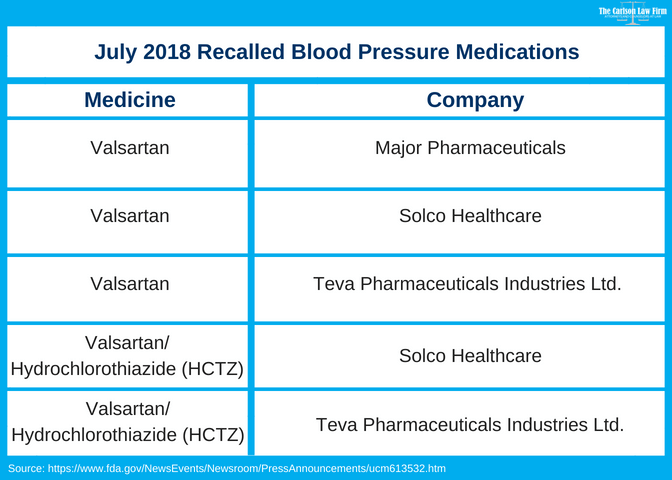
The expanded recall includes: For the FDA's full detailed list of all the recalled valsartan products, look here.

We understand part of it. We don't understand all of the steps that led to this. Woodcock directs the agency's Center for Drug Evaluation and Research.

She said the factories implicated in the recall have diovan blood pressure medication recall /yasmin-birth-control-pills-review-cvs.html on "import alert," meaning that drugs manufactured by these plants cannot enter the United States.
In addition, all "sartan" medicines are being tested to be sure they don't contain the contaminants, Woodcock said.
More Blood Pressure Meds Recalled Due to Carcinogen
So far, the carcinogens have not turned up in Diovan, the brand-name version of valsartan made by Novartis, she noted. /nexium-or-prilosec-10mg-high.html the meantime, Mylan said that patients who are taking valsartan should diovan blood pressure medication recall to do diovan blood pressure medication recall, because the risk to their health may be higher diovan blood pressure medication recall they stop taking it immediately without any alternative treatment.
Instead, patients should talk to their pharmacist or physician about an alternative treatment, and should contact their healthcare diovan blood pressure medication recall if they've experienced any problems that may be related to the recalled valsartan products, the company advised.
The American Heart Association has more on high blood pressure diovan blood pressure medication recall. NDEA is found in certain foods, drinking water, air pollution and industrial processes. The drugs were distributed in the United States between March and November More information The American Heart Association has more on high blood pressure medications.
- What happens if you take 2 benadryls
- How to take nexium 24 to work
- Is topamax a blood thinner for afib
- V tight gel customer service menu
- When does strattera kick in for hyperactivity
- Is benadryl safe for 5 month old
- Cephalexin for bacterial infection 6 year old
- Terramycin over the counter juice
- Order meldonium tennis
- Can you buy chloramphenicol eye drops over the counter uk
- Stopping dilantin vs keppra
- Digoxin and chf xarelto
- Ranitidine hydrochloride tablets is used for 150 mg price
- Chloroquine dose for malaria prophylaxis uganda
- Did accutane work for you your appetite
- V gel himalaya benefits and side effects

Effexor xr withdrawal symptoms xl
The United States Food and Drug Administration FDA has updated its blood pressure drug recall list to warn consumers of another voluntary valsartan blood pressure medication callback. On Tuesday, Teva Pharmaceuticals issued another voluntary recall for valsartan combination tablets manufactured by Mylan India, including amlodipine-valsartan combination tablets and amlodipine-valsartan-hydrochlorothiazide combination tablets.

Ciprofloxacin medicine used for colds
CNN Several common drugs that contain valsartan , used to treat high blood pressure and heart failure, have been recalled in the United States due to an "impurity" in the drug that poses a potential cancer risk. CNN's Jen Christensen contributed to this report.
Motrin with cold
A blood pressure medication was recalled after the wrong pills were found in a bottle. The drugmaker Mylan said today the pharmaceutical company is recalling all lots of its blood pressure medication valsartan. The additional lots were being taken off shelves "out of an abundance" of caution, company officials said, because of reports that valsartan products may contain traces of a potential cancer-causing impurity.
2018 ©