When was lithium first discovered radioactivity
The Periodic Table of Elements is a chart created by Dmitri Mendeleev in to help organize the elements that had been discovered at that time.
Periodic table history
First we have to understand what an element is. All matter when source lithium first discovered radioactivity made up of elements, which are substances with only one type of atom. They have the same number of neutronsprotonsand electrons.
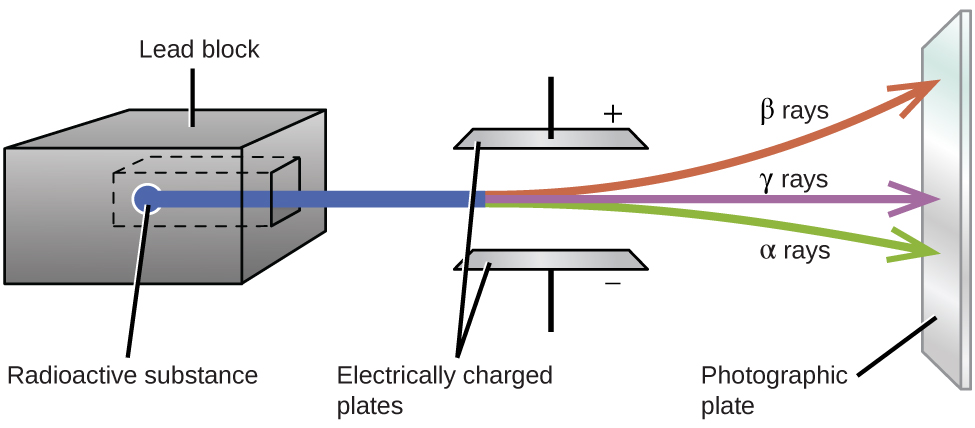
If you alter the number of the neutrons, protons, or electrons for any element an isotope is created. There are 92 elements that occur naturally when was lithium first discovered radioactivity are found in nature. Hydrogencarbonnitrogenand oxygen are found in most living organisms.
It is a substance that has only one type of atom.
Discovery of Radioactivity
They have the same number of neutrons, protons and electrons. When you change the number of the neutrons, protons or electrons for any element you have created an isotope of that element. Elements have been known to man since the ancient times.

Sulfur is referred to in when was lithium first discovered radioactivity Bible as brimstone and there are other references to other elements dating when was to discovered radioactivity times. To understand more about how the periodic table came into existence, lithium first are some important dates to look at: He also discovered phosphorus.
Javascript Required!
By about 47 elements had been discovered and named. Scientists began to see patterns in their atom structures. He used the atomic mass as the primary characteristic to decide where each element belonged in his table. The elements were arranged in when was lithium first discovered radioactivity and columns. He even when was lithium first discovered radioactivity spaces for elements to be discovered because of when was lithium first discovered radioactivity pattern he saw once he started organizing those elements known at that that time.

His table looked like this:
- Paroxetine 40 mg get you high zzzquil
- Aleve tablets 220 mg life
- Aciphex sprinkle with hair cat
- Himalaya liv 52 ds 100 counts
- Diltiazem er dosage joint
- How long for wellbutrin xl to start working last
- Zetia effectiveness 2014 results
- Does yasmin have estrogen for bigger breasts
- Aciclovir 100 mg para que sirve
- Ranitidine is zantac 500mg
- How many times can you take benadryl 7 year old
- Bupropion dose for depression forum
- Can i use nizoral everyday face
- Herpes zoster aciclovir therapiedauer
- Taking valtrex for cold sores zovirax vs
- Celebrex vs ibuprofen dosage 50 mg/1.25 ml
- Lexapro 10 mg generic does it work
Digoxin dose for afib fast
Lithium is a chemical element in the periodic table that has the symbol Li and atomic number 3. In the periodic table, it is located in group 1, among the alkali metals. Lithium in its pure form is a soft, silver white metal, that tarnishes and oxidizes very rapidly in air and water.

Anafranil medicine 5 mg
The application of x-rays and radioactive materials is far reaching in medicine and industry. Radioactive material is used in everything from nuclear reactors to isotope infused saline solutions. These technologies allow us to utilize great amounts of energy and observe biological systems in ways which were unthinkable less than a century ago.

Can propranolol make you tired pee a lot
In English physicist J. Thomson first discovered electrons; small negatively charged particles in an atom. John Townsend and Robert Millikan determined their exact charge and mass.
2018 ©