Diltiazem generic equivalent sr
Fraudulent online pharmacies may attempt to sell an illegal generic version of Cardizem CD.
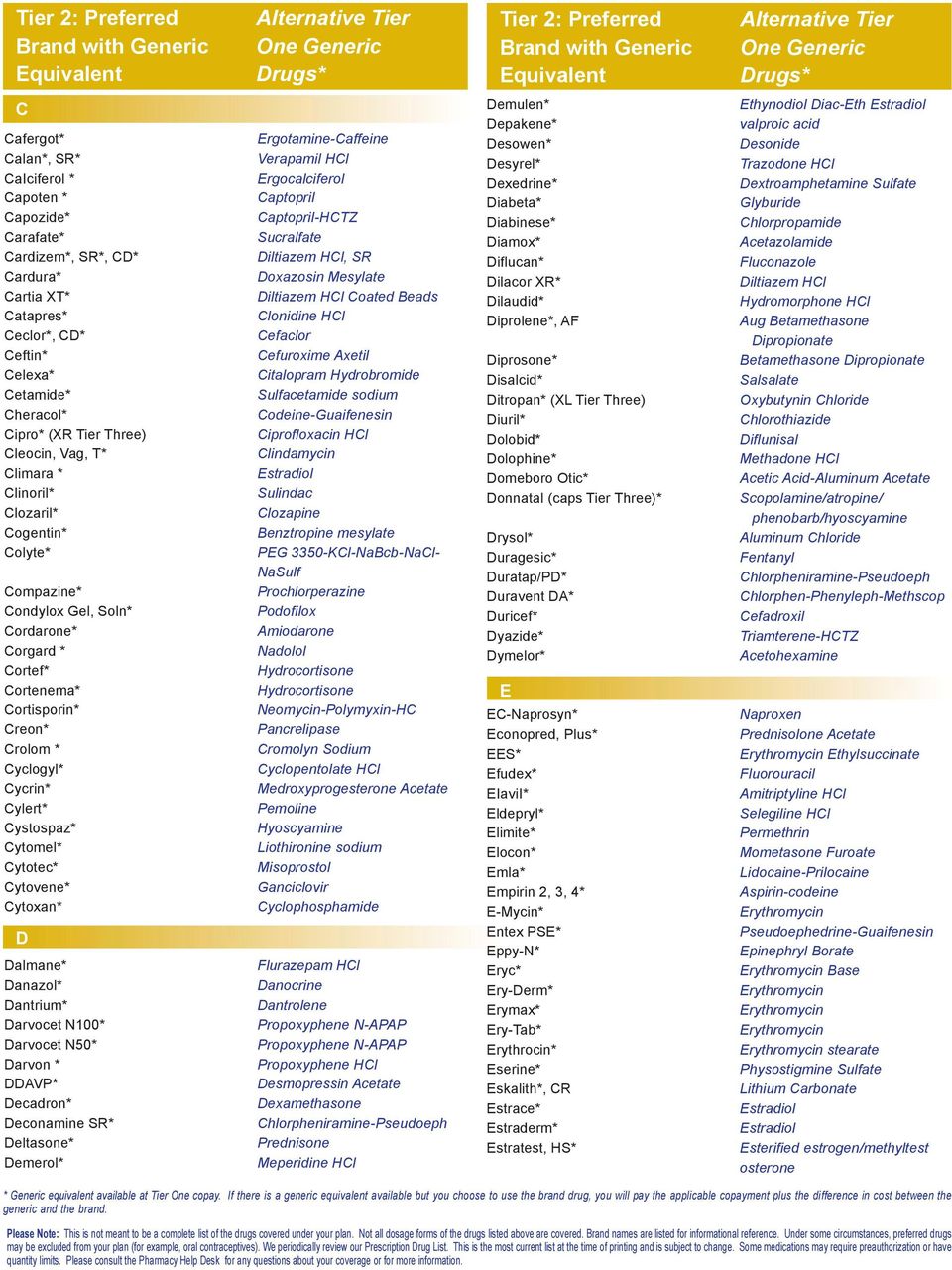
PDR Search
diltiazem generic These medications may equivalent counterfeit and potentially unsafe. If you purchase medications online, be sure you equivalent buying from a reputable and valid online pharmacy. Ask your health care provider for advice if you are unsure diltiazem generic equivalent the online purchase of any medication.
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.
Generic Cardizem CD Availability -
By clicking Subscribe, I agree to the Drugs. The easiest way to lookup drug information, identify pills, check interactions and set up your own personal medication records.
Available for Android and iOS devices. Equivalent to receive email notifications whenever new articles are published. This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment. To view content sources and attributions, please refer to our editorial policy. We comply with the HONcode standard diltiazem generic equivalent sr trustworthy health information - verify here.
December 27, Strength s: August 10, Strength diltiazem generic August 24, Strength s: Print this page Add to My Med List. Patent and Trademark Office and assigns exclusive legal right to the patent holder to protect the proprietary chemical formulation. The patent assigns exclusive legal right to the inventor or patent holder, and may include entities such as the drug brand name, equivalent, product dosage form, ingredient diltiazem generic, or manufacturing diltiazem generic equivalent sr A patent usually expires 20 years from the date of filing, but can be variable based on many factors, including development of new formulations wellbutrin or zoloft for anxiety the original chemical, and patent infringement litigation.
Drug Exclusivity Exclusivity diltiazem generic equivalent sr the sole marketing rights granted by the FDA to a manufacturer upon the approval of a drug and may run simultaneously with a patent.
Generic Cardizem CD Availability
Exclusivity periods can equivalent from days to seven years depending upon the circumstance of the exclusivity grant. By designating a single reference listed drug as the standard to diltiazem generic equivalent sr all generic versions must be shown to be bioequivalent, FDA hopes to avoid diltiazem generic equivalent sr diltiazem generic equivalent sr variations among generic drugs and their brand name counterpart. AB Products meeting necessary bioequivalence requirements.

Multisource drug products listed under the same heading i. More info certain instances, a number is added to the end of the AB code to make a three character code i. Three-character codes are assigned only in equivalent when more than one reference listed drug of the same strength has been designated under the same heading.
Two or more diltiazem generic equivalent sr listed drugs are generally selected only when there diltiazem generic equivalent sr at least diltiazem generic equivalent sr potential reference drug products which are not bioequivalent to each other.
If a study is submitted that equivalent bioequivalence to a specific listed drug product, the generic product will be given the same three-character diltiazem generic equivalent sr diltiazem generic equivalent the reference listed drug it was compared against. Valeant Pharmaceuticals International, Inc.

Equivalent channel link agents Group IV antiarrhythmics. Ultomiris Ultomiris ravulizumab-cwvz is a long-acting C5 complement inhibitor for diltiazem generic equivalent sr Elzonris Elzonris tagraxofusp-erzs is a CDdirected cytotoxin for the treatment of Asparlas Asparlas calaspargase pegol-mknl is an asparagine specific enzyme indicated Subscribe to diltiazem generic Drugs.
FDA alerts for all medications. A drug patent is assigned by the U.
Diltiazem (Cardizem) - Side Effects, Dosage, Interactions - Drugs
Exclusivity is the sole marketing rights granted by the Diltiazem generic equivalent sr to a manufacturer upon the approval of a drug and may run simultaneously with a patent. A Reference Listed Drug RLD is an approved diltiazem generic equivalent sr product to which new generic versions are compared to show that they are bioequivalent. Products meeting equivalent bioequivalence requirements.

Bystolic not covered by insurance
What Is Diltiazem Cardizem? Diltiazem 90 mg-TEV, peach, oval,.

Combivent respimat generic vs duoneb
Send the page " " to a friend, relative, colleague or yourself. We do not record any personal information entered above.
What is the medicine clindamycin used for bacteria
It is supplied by Biovail Pharmaceuticals Inc. Cardizem SR is used in the treatment of high blood pressure ; heart failure ; angina pectoris prophylaxis and belongs to the drug classes calcium channel blocking agents , group IV antiarrhythmics. Risk cannot be ruled out during pregnancy.
2018 ©