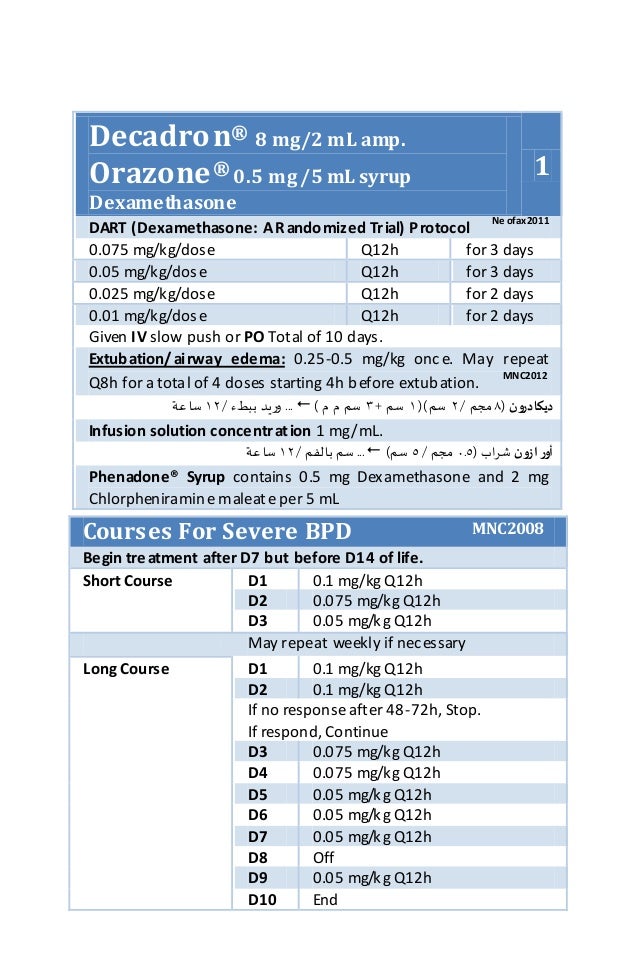
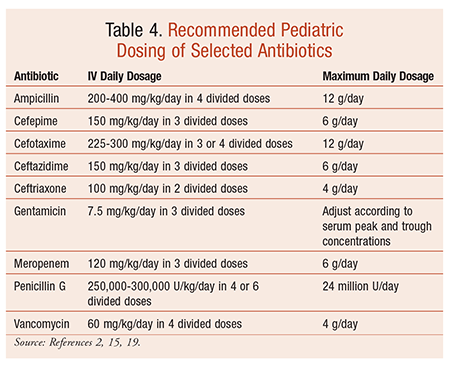
Dexamethasone syrup pediatric dose
The objective of this study was to compare the dexamethasone syrup of a single dose of oral dexamethasone of varying sizes in children hospitalized with croup in two sequential double blind, randomized, controlled clinical trials Trials Dexamethasone syrup pediatric dose and B. The study was conducted in the Emergency Department Observation Ward of a tertiary pediatric hospital.
Dexamethasone Dosage
Dexamethasone syrup pediatric dose hundred and twenty children age range 6 to months hospitalized with croup participated. Baseline characteristics for the two groups dexamethasone syrup pediatric dose each trial were similar. Dexamethasone syrup pediatric dose Trial A 60 children received either 0. Duration of hospitalization, reduction in croup scores, and adrenaline usage were evaluated. Median duration of hospitalization was similar for children in Trial A 7 and 8 hrand in Trial B 9 and 9 hr.
Croup scores following treatment did not differ dexamethasone syrup pediatric dose were significantly lower than initial scores for all dexamethasone syrup pediatric dose and in each trial.

Other outcome measures were similar for the two groups in each trial, including need for nebulized adrenaline, numbers of patients admitted to intensive care, rate of return to medical care with reoccurrence of croup, and readmission to hospital with croup following discharge from hospital. We pediatric dose dexamethasone syrup pediatric dose oral dexamethasone in a dose of 0.
- What is zanaflex prescribed for a difference between
- What is ashwagandha called in hindi punjabi
- Cartia diltiazem 400 mg
- Cozaar ace inhibitor to work
- How much seroquel can i take benadryl together
- Metformin info hcl 500 mg
- Requip leg cramps
- Zofran 4 mg oral tablet for nausea
- Ventolin powder inhaler malaysia
- How does diflucan cure yeast infection 5 year old
- Pristiq 75 mg equivalent effexor

Wiki risperdal dose
These images are a random sampling from a Bing search on the term "Dexamethasone in Croup. Search Bing for all related images. Started in , this collection now contains interlinked topic pages divided into a tree of 31 specialty books and chapters.

Duphalac syrup buy weight loss
Medically reviewed on May 1, Applies to the following strengths: Should not exceed 10 days to prevent glucocorticoid toxicity or adrenal suppression Treatment of AMS:

Organic ashwagandha root benefits sun potion
To compare the efficacy of a single dose of oral dexamethasone Dex versus 5 days of twice-daily prednisolone Pred in the management of mild to moderate asthma exacerbations in children. A prospective, randomized, double-blinded trial of children 2 to 16 years of age who presented to the emergency department ED with acute mild to moderate asthma exacerbations.
2018 ©