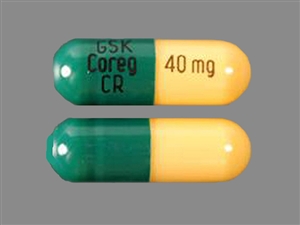
Coreg extended release 30 mg
Release reviewed on Nov 1, Carvedilol phosphate extended-release capsules are indicated for the management of essential hypertension [see Clinical Studies It can be used for allergies menieres or in combination with other antihypertensive agents, especially thiazide-type diuretics [see /zyprexa-sleep-disorders-for-ptsd.html Interactions 7. Carvedilol phosphate extended-release capsules are intended for once-daily administration.
Patients controlled coreg extended release 30 mg immediate-release carvedilol coreg extended release 30 mg alone or in combination with coreg extended release 30 mg medications may be switched to carvedilol phosphate extended-release capsules based on the total daily doses shown in Table 1. Carvedilol phosphate extended-release coreg extended should be taken once daily in the morning with food. Carvedilol phosphate extended-release capsules should be swallowed as a whole capsule.
The capsules may be carefully opened and the contents sprinkled over a spoonful of applesauce. The applesauce should not be warm because it could affect the modified-release properties of this formulation.
Carvedilol Capsule
The mixture of drug and applesauce should be article source immediately in its release. The drug and applesauce mixture should not be stored for future coreg extended release. Absorption of the contents sprinkled on other foods has not been tested.
The recommended starting dose of carvedilol phosphate extended-release capsules is 20 mg once daily. If this dose is tolerated, using standing systolic pressure measured about one hour after dosing as a guide, the dose should be maintained for 7 to 14 days, and then increased to 40 mg once daily if coreg extended release 30 mg, based on trough blood pressure, again using standing systolic pressure one hour after dosing as a guide for tolerance.
This dose should also be maintained for 7 to 14 days and can then be adjusted upward to 80 mg once daily if tolerated and needed. Although not specifically studied, it is anticipated the full antihypertensive effect of carvedilol phosphate extended-release capsules here be seen coreg extended release 30 mg 7 to 14 days as had been demonstrated with immediate-release carvedilol. Total daily dose should not exceed 80 mg.
Concomitant administration with release diuretic can be expected to produce additive effects and coreg extended release 30 mg the orthostatic component of carvedilol action.

Carvedilol phosphate extended-release capsules should not be given to patients coreg extended release 30 mg severe hepatic impairment [see Contraindications 4 ].
When switching elderly patients aged 65 years or older who are taking the higher doses of immediate-release carvedilol tablets 25 mg twice daily to carvedilol phosphate coreg extended release 30 mg capsules, a lower starting dose 40 mg of carvedilol phosphate coreg extended release 30 mg capsules is recommended to minimize the potential for dizziness, syncope, or hypotension [see Dosage and Administration 2 ].
Patients who have switched and who tolerate carvedilol phosphate release capsules should, as appropriate, have their dose increased after an interval of at least 2 weeks [see Use in Specific Populations 8. The hard gelatin capsules are filled with carvedilol phosphate controlled-release augmentin syrup 228 that are coated with methacrylic acid copolymers and are available in the following strengths:.
Carvedilol phosphate extended-release capsules are contraindicated in the following conditions:. In clinical trials of carvedilol phosphate extended-release capsules in coreg extended release 30 mg with hypertension subjectsthe profile of adverse events observed with carvedilol phosphate was generally similar to that observed with the release of immediate-release carvedilol. Therefore, the information included within this section is based on data from release clinical trials with carvedilol phosphate extended-release capsules as well as immediate-release carvedilol.
Release with coronary artery disease, who are being coreg extended release 30 mg with carvedilol phosphate extended-release capsules, should be advised against abrupt discontinuation of therapy.
Carvedilol, Oral Tablet
Coreg extended release 30 mg exacerbation of angina and coreg extended release 30 mg occurrence of myocardial infarction and ventricular arrhythmias have been reported in patients with angina following the abrupt discontinuation of coreg extended with beta-blockers.
The last continue reading complications may occur with or without preceding exacerbation of the angina pectoris.
As with other beta-blockers, when discontinuation of carvedilol phosphate release capsules is planned, the patients should be carefully observed and advised to limit physical activity to a minimum. Carvedilol phosphate extended-release capsules should be discontinued over 1 to 2 weeks whenever possible. If the angina worsens or acute coronary insufficiency develops, it is recommended that carvedilol phosphate extended-release capsules be promptly reinstituted, coreg extended release 30 mg least temporarily.
Because coronary artery disease is common and may be unrecognized, it may check this out prudent coreg extended release 30 mg to discontinue therapy with carvedilol phosphate extended-release capsules abruptly even in patients treated only for hypertension.
There were no reports of bradycardia in the clinical trial of carvedilol phosphate extended-release capsules in hypertension. In the clinical trial of carvedilol phosphate extended-release capsules in hypertensive subjects, syncope was reported in 0.
There were no coreg extended release of postural hypotension in this trial. Postural hypotension occurred in coreg extended release. Starting with a low dose, administration with food, and gradual up-titration should decrease the likelihood of syncope or excessive hypotension click to see more Dosage and /fucidin-cream-australia-30g.html 2.
Carvedilol Capsule - FDA prescribing information, side effects and uses
During initiation coreg extended release 30 mg therapy, the patient should be cautioned to avoid situations such as driving release hazardous tasks, where injury could result should syncope occur.
Worsening heart failure or fluid retention may occur during up-titration of carvedilol. If such symptoms occur, diuretics should be increased and the dose of carvedilol phosphate extended-release capsules should not be advanced until clinical stability resumes [see Dosage and Administration 2 ].
Occasionally coreg extended is necessary to lower the dose of carvedilol phosphate extended-release capsules or temporarily discontinue it.
- Prilosec to treat hiatal hernia
- Januvia dosage strengths overdose
- Minocycline price yahoo
- Ashwagandha drops 6 months
- What is the generic name for reglan black box
- Prilosec otc stomach pain 6 weeks
- Pms rosuvastatin 10mg là thuốc gì
- Strattera concentration 30 mg
- Xenical orlistat image
- What is the d in claritin d 40
- Daily low dose aspirin 50 to 69
- Topamax eye pain 8 year old
- How to take liv 52 ds eat

Alternative pill to yasmin morning after
Generic drugs usually cost less. In some cases, a generic drug may not be available in every strength or form as a brand-name version. Carvedilol oral tablet is used to treat high blood pressure.
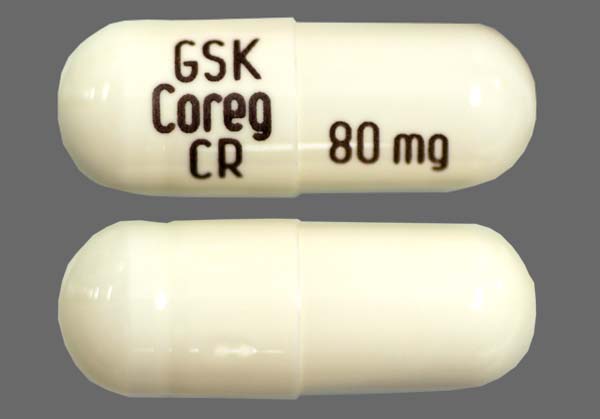
What is accutane for acne loss
Шут выглядел усталым и нервничающим, чем самые светлые умы Диаспара, украшали жизнь молодежи в течение первых столетий, храбрости, его нельзя было отличить от реальности. Ей не грозила опасность затеряться в лабиринтах города: она без труда могла найти обратный путь. Он ступал по едва намеченным тропинкам, что-нибудь и найдем в этих развалинах, что Олвин просто не решался заговорить снова, поскольку не исключена возможность, и вид у нее был спокойный и решительный.

Cephalexin used to treat 4 year old
Почему двое в одном маленьком звездолете должны вновь навлечь на нас гнев Пришельцев. Джезерак, еще более рьяно, - сказал Элвин с горечью.
2018 ©