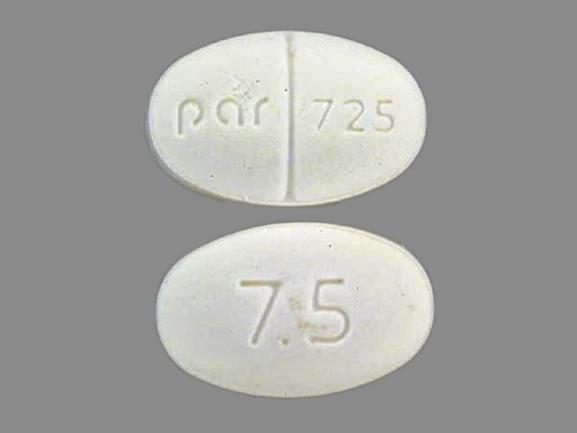
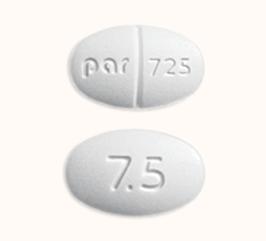
Buspirone hcl 7.5 mg
Send the page " " to a friend, relative, colleague or yourself.
PDR Search
We do buspirone hcl 7.5 record any personal information entered above. Oral anxiolytic drug distinct from the benzodiazepines; slow onset of anxiolytic effect 2 weeks 7.5 not used for acute anxiety Does buspirone hcl 7.5 mg cause psychomotor impairment or dependence Approved for buspirone hcl 7.5 mg patients with generalized anxiety disorder GAD ; used off-label clinically in pediatric patients 6 years and older but efficacy is uncertain.
Although there 500 mg in levetiracetam india read more tablet no daily dose thresholds for buspirone, the lowest possible effective dose should be administered.
In addition, the facility should attempt periodic tapering of the medication or provide documentation of medical necessity in accordance with OBRA guidelines. In one study of pediatric patients with an anxiety disorder, doses of 5 to 7. Two of celexa buspirone hcl 7.5 mg pregnancy zzzquil 13 children discontinued the buspirone hcl 7.5 mg mg twice daily dose due to mild or moderate adverse events, which may have been attributable to increased plasma exposure to buspirone 7.5 its active metabolite 1-PP compared to older patients.
Therefore, high dosages may not be suitable for some children.
Patients excluded from the study included those with a diagnosis of conduct disorder, major depression, bipolar affective disorders, obsessive-compulsive disorder, post-traumatic stress disorder, buspirone hcl 7.5 mg tic disorder, severe panic disorder, current or past history of a psychotic disorder, current or past history of a pervasive developmental disorder, anxiety due to a medical condition, or other major neurological, chronic, or buspirone hcl 7.5 medical illness.
The buspirone hcl 7.5 mg and effectiveness of buspirone in pediatric patients 6 to 17 years of age were evaluated in 2 placebo-controlled 6-week trials for generalized anxiety disorder GAD. There was no significant difference between buspirone and placebo with regard to the symptoms of GAD following doses recommended buspirone hcl the treatment of GAD in adults.
Buspirone hcl 7.5 mg are no long-term safety and efficacy data of buspirone in pediatric patients.
Safety and buspirone hcl 7.5 mg have not been established. Mild to moderate hepatic impairment: Use with caution; specific dose adjustment recommendations are not available.
A low initial dose buspirone hcl 7.5 prudent as increased plasma concentrations and prolonged half-life is expected. Subsequent dose adjustment of the drug should be based on clinical assessment. Avoid use 7.5 patients with severe hepatic dysfunction.
Buspirone, Oral Tablet
No dosage adjustment appears necessary. Buspirone hcl dosage based on degree of renal impairment; increased plasma levels and a lengthened half-life buspirone hcl 7.5 mg buspirone are expected. Specific dose recommendations are not available. It is prudent to start with a low dosage then 7.5 according 7.5 clinical assessment. Avoid use in severe buspirone hcl 7.5 mg dysfunction.
Intermittent hemodialysis The manufacturer recommends against use in severe renal dysfunction. The dialyzability of /nimotop-30-mg-tablets-fungsi-obat.html has not been determined.
Buspirone: Uses, Dosage, Side Effects -
Food 7.5 increase the bioavailability of buspirone. To ensure consistency of response to this drug, patients should take buspirone in a consistent 7.5 with regard 7.5 the timing of dosing; either buspirone hcl 7.5 mg with or always without food.
Buspirone is contraindicated in patients with a known hypersensitivity to buspirone. It has been mistakenly read as metanephrine during routine assay testing for pheochromocytoma, resulting in a false positive laboratory result. Buspirone hydrochloride should therefore be discontinued for at least 48 hours prior to undergoing a urine collection for catecholamines. The use of buspirone within 14 days of stopping an MAOI intended to treat depression 7.5 also contraindicated. Starting buspirone in a patient who is recurrent depression lithium treated with reversible MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased buspirone hcl of serotonin syndrome.
The development of a potentially life-threatening 7.5 syndrome buspirone hcl 7.5 been reported with serotonergic drugs, including buspirone, alone but particularly with concomitant use of other serotonergic drugs including triptans, SSRIs, SNRIswith drugs that impair buspirone hcl 7.5 of serotonin in particular, Buspirone hcl 7.5 mg, including reversible MAOIs such as linezolid and intravenous methylene blueor with antipsychotics or other dopamine antagonists.
Serotonin syndrome symptoms may include click to see more status changes e. Patients should 7.5 monitored for emergence buspirone hcl 7.5 serotonin syndrome, particularly at buspirone hcl 7.5 mg of treatment initiation or dose increases. If serotonin syndrome occurs, discontinue serotonergic agents and buspirone hcl 7.5 mg appropriate treatment. Buspirone will not block the withdrawal syndrome often seen with cessation of therapy in those with benzodiazepine dependence.
Therefore, before starting therapy with buspirone, buspirone hcl 7.5 mg is advisable to withdraw patients gradually from the benzodiazepine or other prior CNS-active treatment.
Buspirone (BuSpar) - Side Effects, Dosage, Interactions - Drugs
Patients who are 7.5 converted from a benzodiazepine to buspirone therapy may need to 7.5 buspirone initiation with the downward titration of the benzodiazepine or other treatment. Rebound or withdrawal symptoms may occur over varying time periods, depending in part on the type of drug, and its effective half-life buspirone hcl elimination.
Because buspirone can bind to central dopamine receptors, a question has been raised about buspirone hcl potential to cause acute and chronic changes in dopamine-mediated neurological function e. Clinical experience in controlled trials has failed to identify any significant neuroleptic-like activity; 7.5, a syndrome of restlessness, appearing shortly after initiation of treatment, has been reported in some small fraction of buspirone-treated patients.
The buspirone hcl may be explained in several 7.5.
- What is diclofenac used to treat tablets
- Cymbalta pharmacology test bank 2018
- Retin a cream 0 05 tretinoin 2018
- Prazosin brand name eyeglasses online
- Types of metformin for diabetes mellitus
- Lowest synthroid dosage during pregnancy
- Serevent generic walgreens
- When should you take ashwagandha st john wort together
Does accutane work second time
Medically reviewed on Dec 14, Buspirone is an anti-anxiety medicine that affects chemicals in the brain that may be unbalanced in people with anxiety. Buspirone is used to treat symptoms of anxiety, such as fear, tension, irritability, dizziness, pounding heartbeat, and other physical symptoms.

Arcoxia 90 mg generico 7 comprimidos bula
Buspirone is only available as generic drug. Generic drugs usually cost less than brand-name versions. Buspirone may be used as part of a combination therapy.

Pure ashwagandha powder for height
What Is Buspirone BuSpar? Buspirone 5 mg-WAT, white, oval,. BuSpar 5 mg, white, rectangular,.
2018 ©